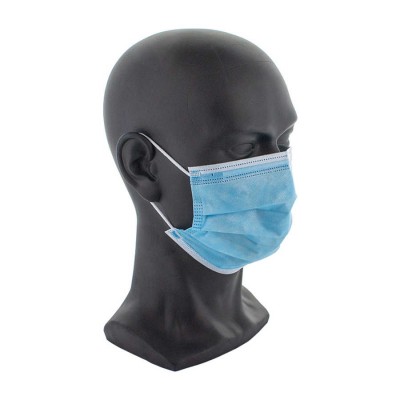
Surgical masks, 3 ply (50), PA126 - PA126
Description
Type IIR medical masks are recommended for healthcare personnel, patients and all others to reduce the spread of infection due to the COVID-19 pandemic.
A Type IIR mask for medical use, compliant with standard EN 14683, thus providing an appropriate antimicrobial barrier, is effective in reducing the release of infectious agents originating from the nose and mouth of a patient or a person presenting clinical symptoms or who is asymptomatic, particularly during an epidemic or pandemic, as is the case with COVID-19.
The standard EN 14683 specifies the manufacturing, design and performance requirements, as well as the test methods relating to medical face masks intended to limit the transmission of infectious agents from members of the medical team to patients during surgical and other medical procedures with similar requirements.
This 3-layer mask for medical use is a medical device, usually consisting of a filter layer positioned, bonded or pressed between layers of non-woven fabric. It must not disintegrate, separate or tear during its intended use.
A Type IIR mask for medical use, compliant with standard EN 14683, thus providing an appropriate antimicrobial barrier, is effective in reducing the release of infectious agents originating from the nose and mouth of a patient or a person presenting clinical symptoms or who is asymptomatic, particularly during an epidemic or pandemic, as is the case with COVID-19.
The standard EN 14683 specifies the manufacturing, design and performance requirements, as well as the test methods relating to medical face masks intended to limit the transmission of infectious agents from members of the medical team to patients during surgical and other medical procedures with similar requirements.
This 3-layer mask for medical use is a medical device, usually consisting of a filter layer positioned, bonded or pressed between layers of non-woven fabric. It must not disintegrate, separate or tear during its intended use.
show more
show less
Product Data (5)
| Brand | Peach |
| Manufacturer | Büttner s.r.o. Tuchorazska 1347, 28201 Cesky Brod, CZ info@buttner.cz |
| Peach Product Code | 511099 (PA126) |
| EAN | 7640460545463 |
More Products

FFP2 Half-mask with filter (25 pcs), PA120

3 x Hygieneschlange - PA122

Hygieneset - PA123

FFP2 Half-mask with filter (1 pcs), PA125/1
Surgical masks, 3 ply (50), PA126 - PA126

Reusable masks (10 pcs), PA127

Waschbare Mund-/Nasenmaske child (10 pcs), PA128

Peach - Nitrile disposable gloves powderfree, latexfree - 100pcs - size S - PA132-S

Peach - Nitrile disposable gloves powderfree, latexfree - 100pcs - size M - PA132-M

Peach - Nitrile disposable gloves powderfree, latexfree - 100pcs - size L - PA132-L

FFP2 Half-mask with filter (20 pcs), PA120

FFP2 Half-mask with filter (1 pcs), PA125/1

Hygieneschlange - PA122

Peach Hand disinfecting wipe, PA130

Loading